NIB is the first institute in the country to initiate testing of COVID-19 suspected clinical specimens on M/s Roche cobas® 6800 system, a fully automated, high-throughput system from 04th April 2020. cobas® SARS-CoV-2 diagnostic kit used on cobas® system is a real time RT- PCR two target test, intended for the qualitative detection of nucleic acids from SARS-CoV-2 in nasopharygeal/ oropharyngeal samples collected in Viral Transport Medium (VTM). cobas SARS-CoV-2 is based on fully automated nucleic acid extraction and purification followed by PCR amplification and detection. The cobas® 6800 System consist of the sample supply module, the transfer module, the processing module, and the analytic module.
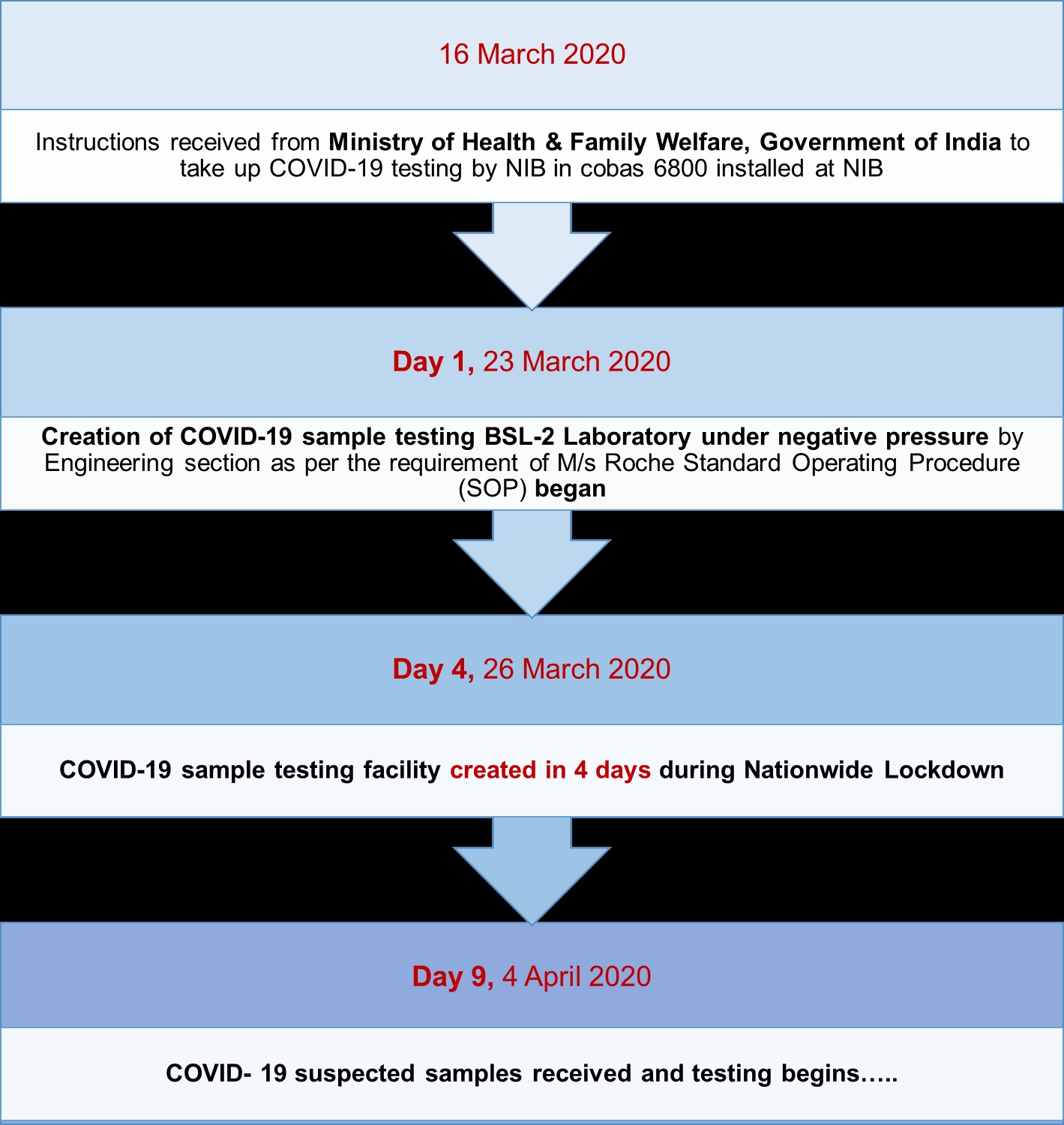
NIB has received COVID-19 suspected samples from various Quarantine camps/ Hospitals located in different districts of the Delhi-NCR, Uttar Pradesh (Aligarh, Bulandshehar, Baghpat Gautam Budha Nagar, Ghaziabad, Hapur, Meerut, Muradnagar, Mujaffarnagar & Saharanpur), Madhya Pradesh (Bhopal & Indore) Rajasthan (Jodhpur) and Ladakh. For smooth functioning of COVID lab, a dedicated sample receiving area, sample logging area, data entry operator room, sample sorting area and bar-coding area to label secondary tubes etc. were set in-place. In addition, a dedicated walk-in cold room at 2-8°C for storing samples was allocated for storage of samples. Proper decontamination and waste disposal SOPs were used. There was restricted entry in these areas and COVID lab was under CCTV coverage.
NIB remains in solidarity in our nation's battle against COVID-19 and is constantly devoted for the cause of public health safety of its compatriots.
